Posted: Feb 14, 2020 12:29 pm
Macdoc wrote:released heat flows to the next evaporating layer,
HOW???!!
Here is the paper
https://pubs.rsc.org/en/content/article ... ivAbstract
That's the question. Searching "recyking enthalpy" turned up this:
https://www.sciencedirect.com/science/a ... 511830388X
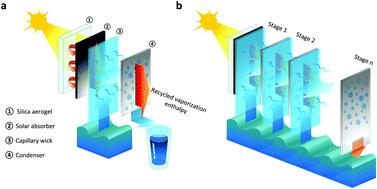
Results and Discussion
The storage and recycling of interfacial solar steam enthalpy for simultaneous generation of clean water and electricity is shown in Figure 1B (the real setup is shown in Figure S1). This high-temperature steam generated by an interfacial solar steam generator (as shown in Figure S1A) flows into the area of thermal storage and water generation, with an aluminum chamber wrapped by a polyurethane foam (as shown in Figure S1B) for thermal insulation. Because of the heat exchange between high-temperature steam and the chamber, steam will be condensed to produce clean water at the outlet of the chamber, while the energy in the steam is reserved in the chamber to maintain it at a high temperature. The temperature gradient formed between this chamber and the room-temperature environment can then be used for electricity generation by using the thermoelectric modules (based on Bi2Te3 materials; for more details see Experimental Procedures).
But I'm still confused how you can condense steam on a hot plate. Don't you need to keep the condenser cold for the system to work?
I'm assuming that it does work, serious institutions are involved so it seem more likely my ignorance than anything else.