Posted: Dec 20, 2014 12:21 pm
BooBoo wrote:Shrunk wrote:so Behe is right to suppose that a simultaneous mutation is likely necessary,
Heh. That's funny. The Casey Luskin article I quote above says that is exactly what Behe is not saying. Luskin is demanding an apology from the people who said that's what Behe was claiming. So are you going to apologize to Behe, like Luskin is demanding?
A simultaneous mutation would *definitely* work. That is the point being made and which nobody can deny. What Behe has long argued is that a single mutation, on its own, is going to be harmful and so will be actively selected against.
And he's wrong. From the Larry Moran article I linked:
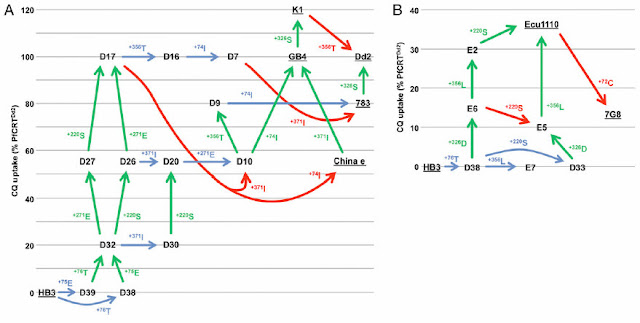
This is where the data in the Summers et al. (2014) paper becomes relevant. They have a nice figure showing how the chloroquine resistant stains arose from a series of strains that acquired different mutations. The seven resistant strains are underlined (e.g. GB4). If you look at the group of strains on the left, you will see that there are two possible mutational routes to strain D32, which is not significantly resistant to chloroquine in the field even though it carries two essential mutations in the PfCRT gene.
In one pathway, the mutation N75E occurs first giving rise to strain D39 and then the K76T mutation occurs in that strain creating D32. The order of the mutations is reversed in the other pathway. In either case, the first mutation has no effect on the chloroquine uptake while the addition of the second mutation produces a significant effect.
It is important for Behe's argument that the "first" mutation is deleterious and he claims that the K76T mutations is, in fact, deleterious on its own. He says ....Close your eyes and envision a pathway to a malaria parasite that has four mutations. The first mutation is deleterious, the second rescues the first and makes the parasite marginally chloroquine resistant. Subsequent steps are all beneficial by dint of either improving chloroquine resistance or of stabilizing the structure of the mutated PfCRT, which is required for malaria survival. Once a parasite can survive at least marginally in the presence of chloroquine, further mutations can be added one at a time (no longer two at a time) in each cycle of infection because the population size (1012) greatly exceeds the inverse of the mutation rate.
In the argot of chemical kinetics, getting beyond the deleterious mutation is the "rate-limiting step." After that hurdle is passed further mutations can be added singly -- the way Darwinists like -- and comparatively rapidly. Since they would be added rapidly, they would be difficult to detect in the wild. Hence the pattern described by Summers et al. fits the scenario I described perfectly.
Assuming that Behe is correct about the K76T strain (this is not certain), then the pathway N75E → K76T → strain D32 does not have an intermediate that is "rate-limiting" because the K76T mutation is never present on its own. It seems to me that ALL the pathways have to have a deleterious intermediate in order for Behe's scenario to make sense. In other words, both N75E and K76T have to be deleterious.
This neutral pathway to resistance has been observed. There's a strain in the wild called 106/1 that contains several different allelic variants in the PfCRT gene but it's sensitive to chloroquine. That strain becomes full-blown resistant to chloroquine in a single step when it acquires the K76T mutation (Cooper et al. 2007). Contrast this with Behe's explanation that requires two mutations to occur together in a single infected individual before you can get a resistant parasite.